Philosophy:
People first. This is the guiding philosophy for our laboratory. Our greatest assets are the people in our lab, our collaborators, and the innovative ideas and enthusiasm they bring to our science. And, of course, the ultimate goal of our efforts is to help people; our patients with severe neurological disease and the loved ones caring for them.
Scientific Mission:
We have developed human stem cell derived brain organoid models that are capable of generating complex physiological activities together with tools to record and quantify these. The core scientific mission of this laboratory is to continue to innovate and expand on what we have already developed while leveraging our current methodologies to dive deeper into the mechanisms of normal neural circuit development and how neurological disease perturbs circuit formation and function.
Overview:
In all projects described below we primarily use organoids generated from iPSCs harboring single gene variants associated with epilepsy and autism. More recently, we have also acquired multiple iPSC lines harboring pathogenic mutations in the microtuble-associated protein tau (MAPT) gene. Patients with this mutation have an early onset Alzheimer’s-like dementia, and we are actively generating organoids harboring this mutation in our studies of hippocampal circuit formation and function.
Examples:
The following represent a few (but not all) of the ongoing projects in the lab. We also spend considerable effort within all of our projects in the refinement and development of our organoid generation techniques and analysis approaches.
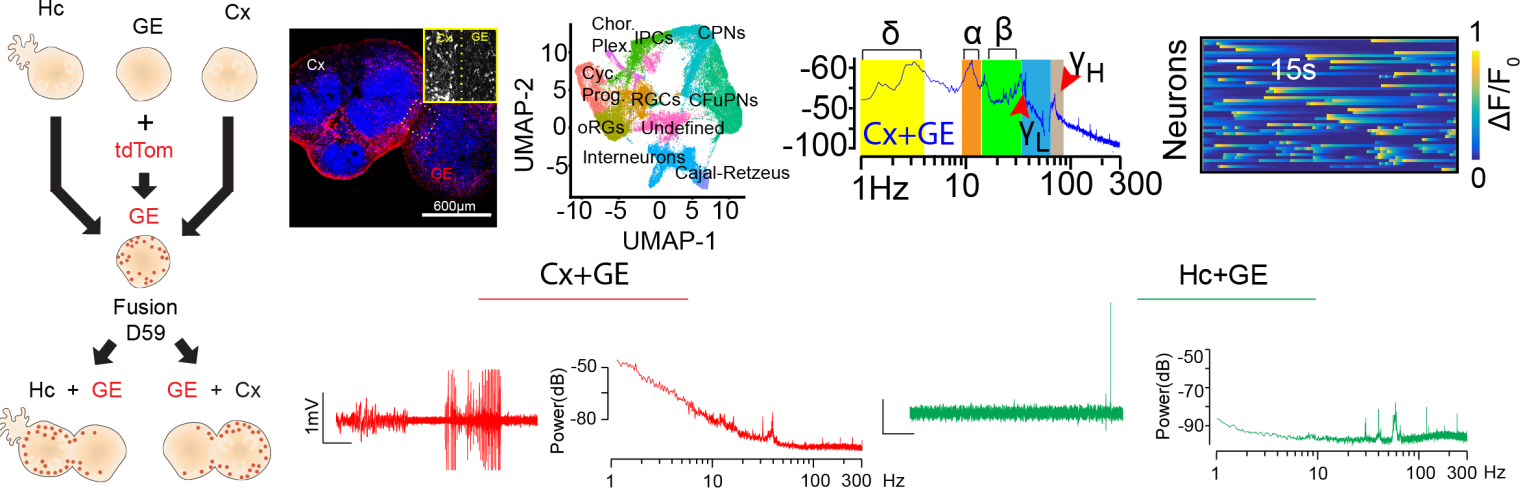
Using brain-region specific organoids to delineate regional divergence in human neural circuit development and function:
Many neurological disorders are associated with impairments in multiple cognitive domains. For example, a patient with a primary diagnosis of autism may also have comorbid epilepsy and intellectual disability. Even in cases where the neurological disorder can be traced to a single gene pathogenic variant it remains unclear if a single upstream pathological driver has shared or unique effects on circuit function in distinct brain regions. This kind of regional divergence may have important implications for both disease pathogenesis and treatment. In recent work, we have focused on the differential impact of single gene variants on the development and function of hippocampal versus forebrain cortical circuits.
Oscillatory Failure in Autism:
Brain oscillations are believed to be critical for neural computation and can serve as a biomarker of excitatory- inhibitory (E/I) balance in neural circuits. Disruptions in oscillatory activity have been observed in patients with autism spectrum disease (ASD). Indeed, oscillatory dysregulation is thought to be a pathophysiological mechanism linking E/I imbalance with cognitive difficulties in ASD. In particular, a failure to generate gamma oscillations in early life is associated with language, cognitive, and attention deficits characteristic of ASDs. We recently developed a novel brain organoid platform ideal for modeling the effect of E/I imbalance on downstream cellular plasticity and oscillatory activity. Specifically, we have generated an organoid system where we can consistently generate gamma oscillations. In preliminary studies, we have shown a loss of gamma oscillatory power in organoids generated from patient induced pluripotent stem cells (iPSCs) harboring autism risk genes. In this project we will use a combination of multiphoton calcium indicator imaging, confocal microscopy, and single cell RNA sequencing to identify the cellular etiology of this change.
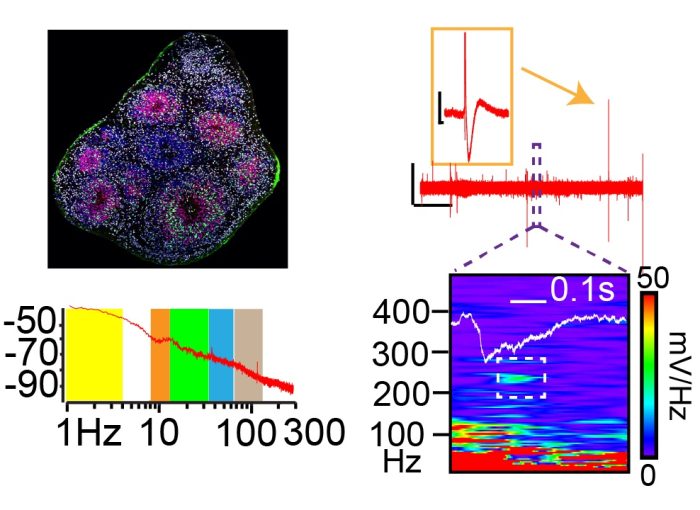
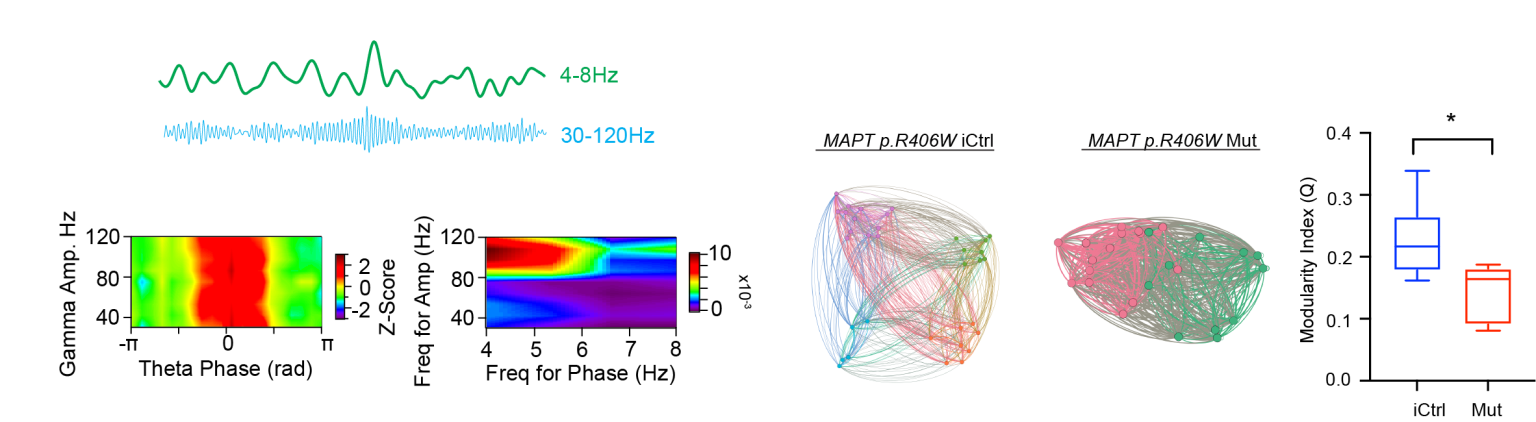
An Organoid Model of Hippocampal Circuit Function:
We have generated hippocampal organoids that are capable of generating stereotyped neural circuit activities associated with learning and memory. Specifically, we have observed patterns of theta-gamma phase amplitude coupling and a specific type of high frequency oscillation called a sharp wave ripple. In ongoing projects, we are exploring how these patterns are altered by single gene pathogenic variants in tau that result in an Alzheimer’s-like dementia.